Abstract
Introduction:
There is increased risk of congestive heart failure (CHF) following anthracycline-based chemotherapy in patients with Diffuse Large B-cell lymphoma (DLBCL). Other than increased cumulative anthracycline dose, little is known about risk factors for developing CHF and other cardiovascular events (CVE).
Methods:
We conducted a retrospective analysis of 539 DLBCL patients diagnosed at University Hospitals Seidman Cancer Center between 2002 - 2016. Baseline patient and disease characteristics, patterns of treatment and outcomes after therapy, including response, survival, relapse, CVE, and causes of death were collected.
Time-to-CVE was measured from the date of DLBCL diagnosis to the date of CVE diagnosis and censored at the date of last follow-up for those without CVE, considering death as competing risk. The cumulative incidence of CVE was estimated taking death as competing risk into account. Univariate and multivariable analysis on time-to-CVE was done using Gray's method. Multicollinearity was tested and some of the highly correlated covariables were excluded in the final analysis. CVE risk scores were computed for each patient as a linear combination of the factors included in the multivariable model and their respective coefficients. The optimal cutoff point was identified by searching the spectrum of the risk scores to have the best discrimination of time-to-CVE among patients at low/high risks. All tests were two-sided and p-value ≤ 0.05 were considered statistically significant.
Results:
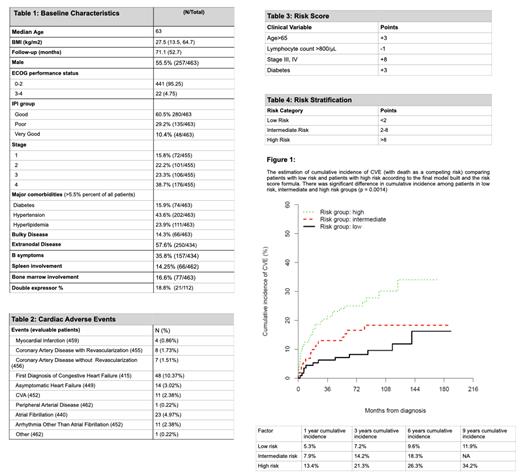
We identified 463 patients who received anthracycline to be included in the statistical analysis. Baseline clinical and disease characteristics including comorbidities, clinical risk factors are outlined in Table 1.
After a median follow up of 71.1 (range: 0.3 - 216.1) months, 5-year PFS was 59% with median PFS of 82.7 (95% Cl: 71.1 - 115.7) months and 5-year OS was 71% with median OS of 152.6 (95% CI: 127.5 - 162) months.
CVE were identified in 92 patients (19.9%), with 128 events identified. The most common CVE was new onset of CHF in 10.4% of patients, followed by atrial fibrillation (4.9%) and asymptomatic heart failure ( 3%) (Table 2).
The 3, 6 and 9-year cumulative incidence of CVE for early stage was 13.1%, 15.2% and 18.5%, respectively and 22.2%, 31.4% and 36.5% for advanced stage, respectively (p-value = 0.026). Similarly, the cumulative incidence of CVE was increased in patients with diabetes, with a 3-year cumulative incidence of CVE of 9.4% vs. 17.5% in patients with and without diabetes, respectively.
Proportional hazards regression for time-to-CVE identified age >65 years, CD10 expression by IHC, diabetes, and hypertension as significant predictors of time-to-CVE on univariate analysis. On multivariable regression analysis with advanced age at diagnosis, stage, baseline lymphocyte count, and diabetes; stage was still significant in predicting time-to- CVE controlling the effects of advanced age and diabetes, which were also marginally significant.
A predictive formula was created to help identify patients at highest risk for cumulative incidence of CVE. Using advanced age (over 65 years of age), baseline lymphocyte count above 800/µL, advanced stage at diagnosis and history of diabetes patients could be stratified into groups with low, intermediate and high risk for CVE (Table 4 and Figure 1).
Risk score = Age >65 (3 points) + Lymphocyte count > 800/µL (-1 point) + Stage III/IV(8 points) + Diabetes (3 points) (Table 3)
One-year cumulative incidence of CVE was 5.3% for low-risk patients, 7.9% for intermediate risk patients and 13.4% for high risk patients (Figure 1).
Conclusions:
Our analysis indicates a high risk of CVE in anthracycline treated DLBCL patients, with 19.9% of patients having a CVE in our cohort. The proposed CVE predictive score uses clinical and laboratory parameters routinely collected on all new DLBCL patients and can be easily assessed in the clinical setting. Patients in the high-risk group have a cumulative incidence of CVE of 13.4% at 1 year and would likely benefit from surveillance and interventions. Use of a clinical risk score can aid in the identification of patients at highest CVE risk to conduct studies of surveillance and prevention of this common complication.
Boughan: Beigene: Speakers Bureau. Caimi: Seattle Genetics: Consultancy; Verastem: Consultancy; Amgen Therapeutics.: Consultancy; Kite Pharmaceuticals: Consultancy; TG Therapeutics: Honoraria; ADC Theraputics: Consultancy, Research Funding; Genentech: Research Funding; XaTek: Patents & Royalties: Royalties from patents (wife).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal